Breadcrumbs
04 Thermal Physics
Thermal Physics. In H2 Physics, the everyday concepts of heat and temperature are reexamined. Single-particle mechanics is applied to model an ideal gas, which is one of the simplest many-body systems. A crucial purpose of this section is to connect the microscopic behaviour of individual constituents with the macroscopic properties of the collective system, for learners to simultaneously see the forest and the trees. The strand of energy provides insight into physical processes like melting and boiling for material substances generalised beyond ideal gases. The overlap with what learners might have encountered in chemistry provides opportunities for teachers to discuss cross-curricular connections.
Big Ideas 1. When a substance gains or loses heat, the substance may change its temperature, change its state, change its volume or change to a different substance. The kinetic model allows us to understand the macroscopic properties of matter and changes in its state in terms of its microscopic molecular structure and behavior. 2. Energy may be transferred through all materials and through free space (vacuum). Our understanding of the different mechanisms of heat transfer through different materials enables us to control and make use of heat transfer in many appliances and machines. 3. The internal energy of a body consists of the total kinetic energy and potential energy of the particles in the body. Changes in a body or a substance due to heat gain or heat loss may be explained by the change in its internal energy. 4. Thermodynamics is the study of the relationship involving heat, mechanical work and other aspects of energy and energy transfer. The first law of thermodynamics is a general statement of the law of conservation of energy that includes energy transfer through heat as well as mechanical work. The ideal gas equation gives the relationship of the pressure, volume, temperature, and the number of moles of a gas. This equation allows us to find the state of a gas in any situation.
- Details
- Written by Shaun
- Parent Category: Physics
- Category: 04 Thermal Physics
- Hits: 2629
Read more: Measuring the Heat Energy Between 2 Cups of Water Javascript HTML5 Applet
- Details
- Written by Loo Kang Wee
- Parent Category: Physics
- Category: 04 Thermal Physics
- Hits: 6230
Read more: Conduction of 3 different materials model by Energy2D
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 03 Temperature
- Hits: 8226
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 03 Temperature
- Hits: 2509
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 03 Temperature
- Hits: 1544
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 2049
- Details
- Written by Shaun
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 5075
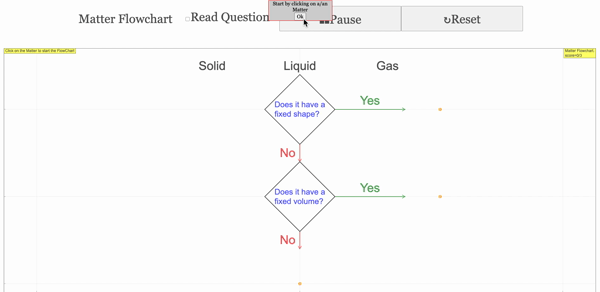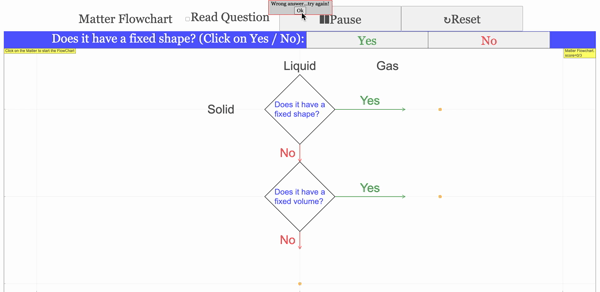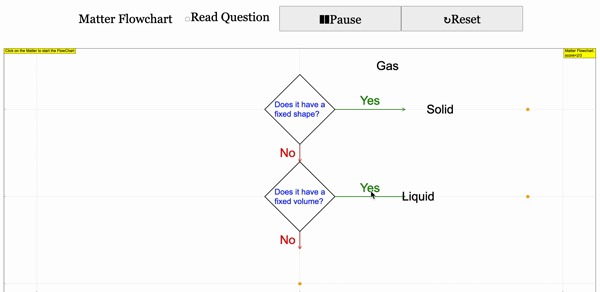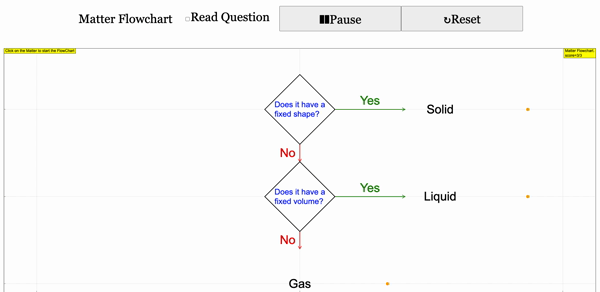
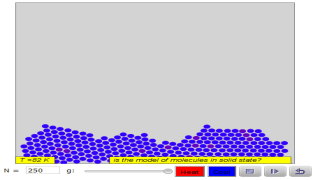
Read more: 3 States of Matter Interactive Flowchart Javascript HTML5 Applet
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 12398
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 13384
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 8736
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 9163
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 12849
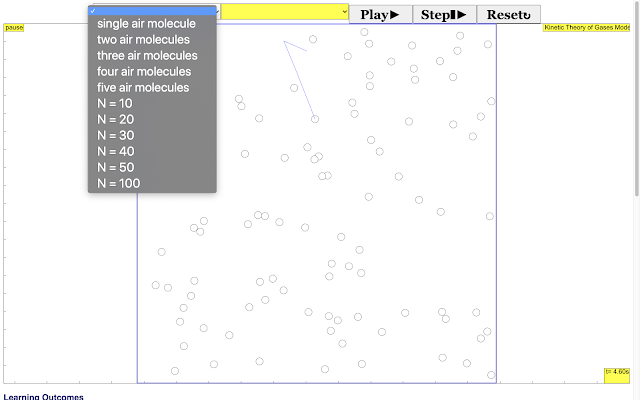
Read more: Three States of Matter JavaScript HTML5 Applet Simulation Model
- Details
- Written by Loo Kang Wee
- Parent Category: 12 Temperature & Ideal Gases
- Category: 01 Kinetic Model
- Hits: 3302
- Details
- Written by keith_zhang_boliang@moe.edu.sg
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 1912
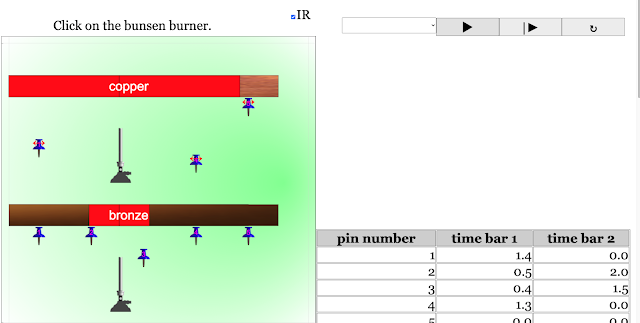
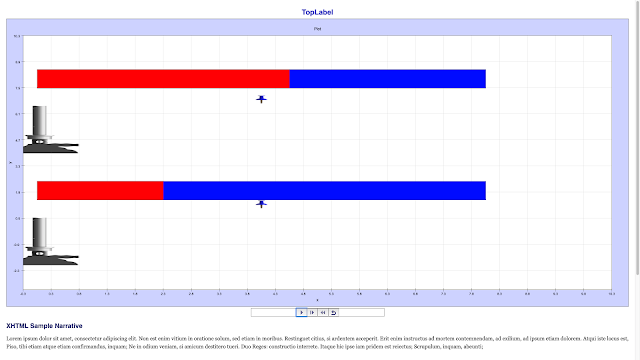
Read more: Transfer of Thermal Energy: Conduction in Metals and Non-Metals
- Details
- Written by Loo Kang Wee
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 4649
- Details
- Written by Loo Kang Wee
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 1943
- Details
- Written by Jonathan
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 5481
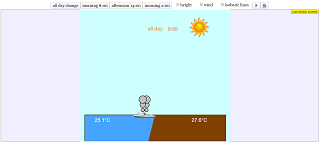
Read more: Convection current JavaScript HTML5 Applet Simulation Model
- Details
- Written by Loo Kang Wee
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 4594
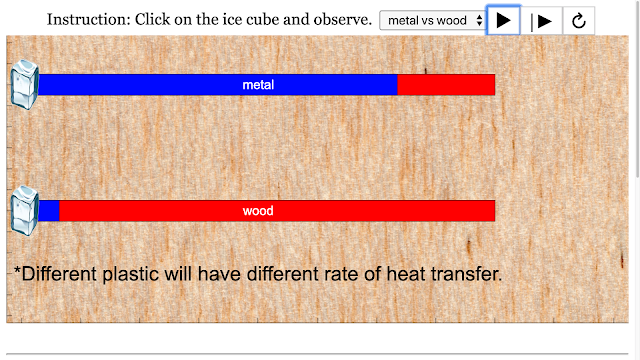
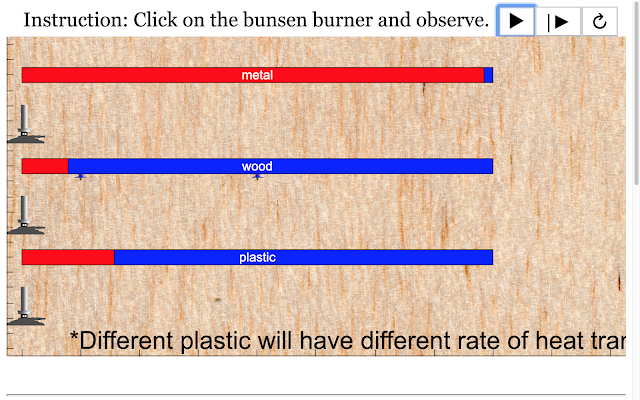
Read more: SLS Hackathon by Yumin Primary on Heat Gain Comparing 2 Materials
- Details
- Written by Loo Kang Wee
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 2831
Read more: SLS Hackathon by Yumin Primary on Heat Loss comparing 2 materials
- Details
- Written by Loo Kang Wee
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 2603
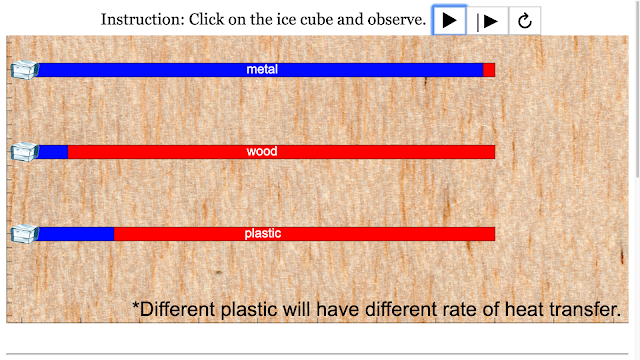
Read more: SLS Hackathon by Yumin Primary on Heat Loss by 3 Materials
- Details
- Written by Loo Kang Wee
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 2670
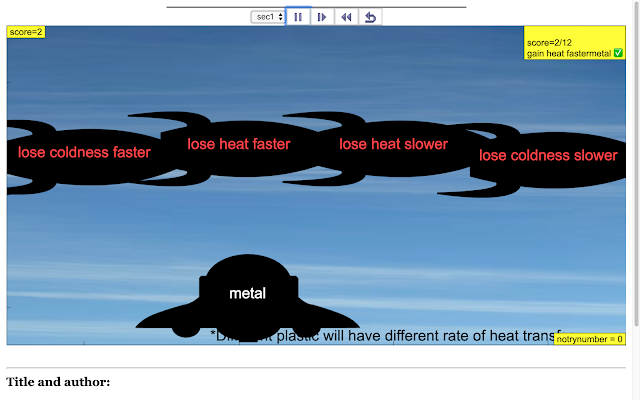
Read more: SLS Hackathon by Yumin Primary on Catch the correct Heat Transfer Game
- Details
- Written by Loo Kang Wee
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 2894
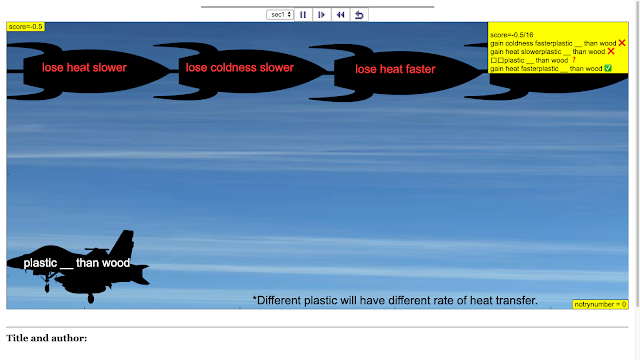
Read more: SLS Hackathon by Yumin Primary on Catch the correct Heat Loss and Gain Game
- Details
- Written by Loo Kang Wee
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 3169
Read more: SLS Hackathon by Yumin Primary on Heat Gain by 3 Materials
- Details
- Written by Loo Kang Wee
- Parent Category: 04 Thermal Physics
- Category: 13 Thermodynamic Systems
- Hits: 2622
Read more: SLS Hackathon Example Heat Transfer on Rods Primary School Game HTML5
- Details
- Written by Shaun
- Parent Category: 13 Thermodynamic Systems
- Category: 04 Thermal Properties of Matter
- Hits: 4959
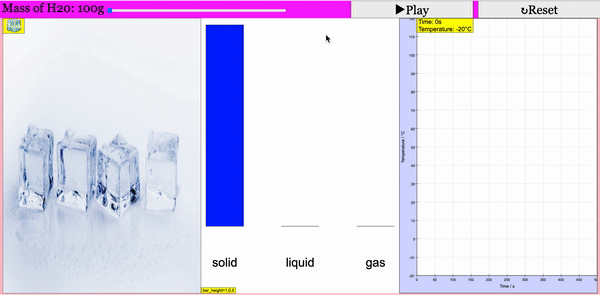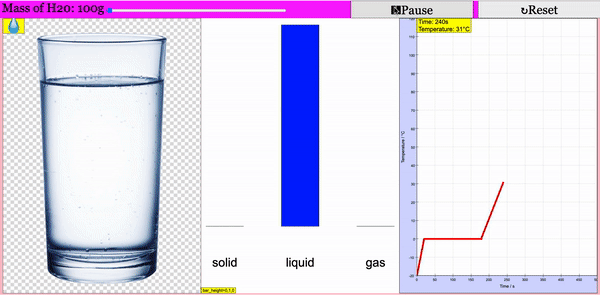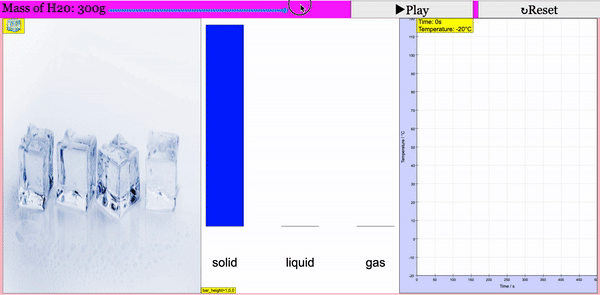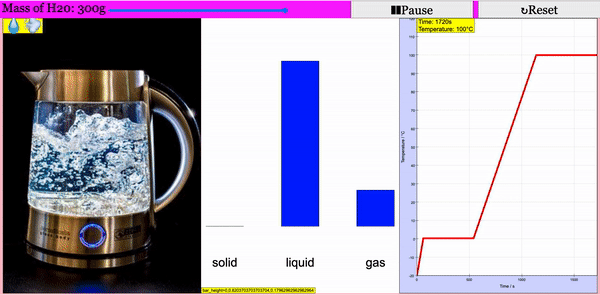
Read more: Adding Heat to Water, Melting and Boiling 2021 JavaScript HTML5 Applet Simulation Model
- Details
- Written by Loo Kang Wee
- Parent Category: 13 Thermodynamic Systems
- Category: 04 Thermal Properties of Matter
- Hits: 2709
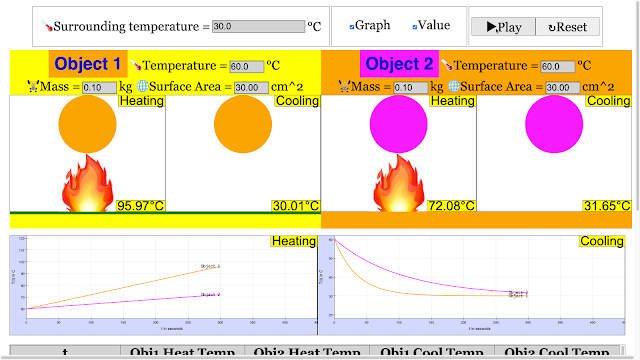
Read more: Cooling and Heating Curve of 2 Objects for Primary School Science HTML5 Simulation
- Details
- Written by Wei Chiong
- Parent Category: 13 Thermodynamic Systems
- Category: 04 Thermal Properties of Matter
- Hits: 5033
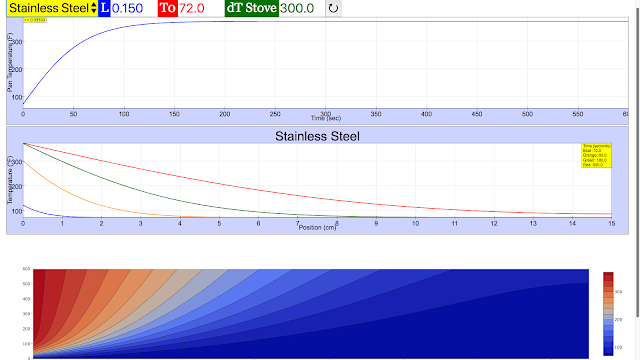
Read more: Steady-State Heat Conduction JavaScript Simulation Applet HTML5
- Details
- Written by Fremont
- Parent Category: 13 Thermodynamic Systems
- Category: 04 Thermal Properties of Matter
- Hits: 4781
Read more: Pressure Volume Diagram JavaScript Simulation Applet HTML5
- Details
- Written by Loo Kang Wee
- Parent Category: 13 Thermodynamic Systems
- Category: 04 Thermal Properties of Matter
- Hits: 3529
Read more: PICUP Heat flow -- Dynamics of a 1D rod JavaScript Simulation Applet HTML5
- Details
- Written by Loo Kang Wee
- Parent Category: 13 Thermodynamic Systems
- Category: 04 Thermal Properties of Matter
- Hits: 7703