Titration Curve of Weak Base against Strong Acid
(image)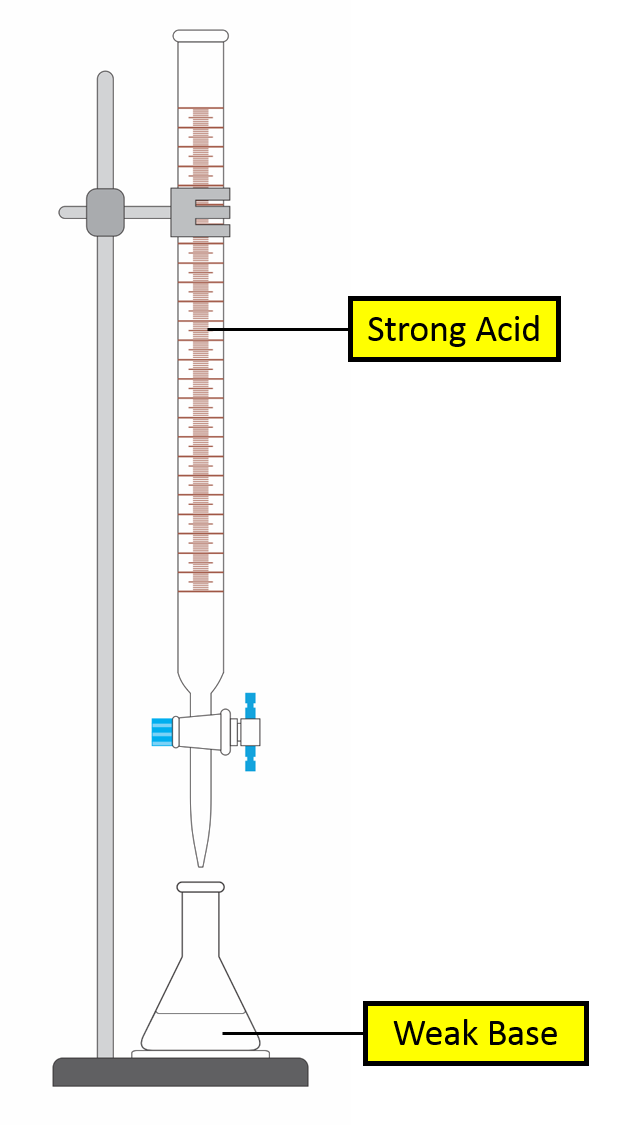
Titration Curve of Weak Base against Strong Acid
(image)
|
Self check 1. Select a point by clicking the graph:Burette Volume pH 2. Identify the species present in the conical flask at that point: WB conj acid of WB xs SA 3. Is solution a buffer? yes |
|
||||||||||||||||||||||||||||||||||||||||||||||||||